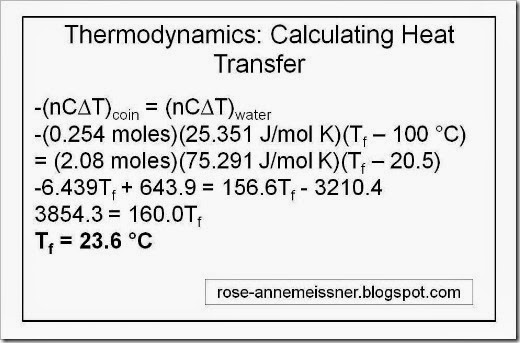
This post will be my first lesson on this site. I offer these lessons as a gift in the spirit of Sacred Economics by Charles Eisenstein. If you’ve arrived at this post, I assume you are looking for some help to better understand the classic phenotypes that are seen in studies of mitotic recombination. In this lesson, I’ll explain the mechanism that may explain one phenotype, twin spots. Here is a very simple graphic to illustrate the twin spots phenotype:

We can see that we have two “spots” on the fly’s back (dorsal surface for you anatomy fans), right next to each other, and each spot shows a different mutant phenotype (which I’ll explain below). Note that the wild-type phenotype is a brownish-beige body and straight-ish bristles.
Mitotic recombination is the phenomenon whereby homologous chromosomes swap portions of DNA with each other in non-meiotic cells. It’s a rare event and often induced either by X-ray radiation or by transgenes that cut and splice DNA when they are expressed. One of the most interesting questions that can be answered by mitotic recombination is whether a phenotype is cell autonomous. In other words, is the phenotype of a cell or patch of cells due to the genetic activity within that cell or patch of cells, or does it depend on the genetic activity of a different cell?
In Drosophila, we can study mitotic recombination using mutant alleles that affect the appearance of the body surface. Dr. Curt Stern did just this using alleles for two genes: yellow (y) and singed (sn)1. Homozygous yellow mutants have a yellow body color, while homozygous singed mutants have bent, funky-looking bristles.
(Check it out: yellow mutants and singed mutants. For the singed mutants, compare D [a wild-type control called Oregon R] to A [a singed mutant].)
Stern worked with female flies that were heterozygous for yellow and singed. These genes are on homologous chromosomes. In Stern’s experiment, the mutations were on different but homologous chromosomes. We can write this genotype like this:

y+ sn- is the genotype of one chromosome, and y- sn+ is the genotype of the other. Heterozygous mutants like this are also called trans-heterozygotes because the mutations are found on different chromosomes.
Most of the animals Stern examined were wild-type in appearance. In other words, their body color and bristles did not show mutant phenotypes. This is what we would expect in animals that have a wild-type copy of each gene. But sometimes he observed what were dubbed “twin spots” where a spot of yellow was adjacent to a spot of singed bristles. This cartoon is a nice illustration of the phenotype. Because of the location of these twin spots, Stern proposed that they were the product of mitotic recombination between the centromere and two loci located on the same side of a chromosome.
So how would that mechanism work? Let’s draw it out and follow the chromosomes through cell division.
First, let’s consider what the chromosomes look like without mitotic recombination. For simplicity, I have omitted everything but the most essential details in the diagrams below. CENT is the centromere of the each chromosome.

Now let’s look at what happens when this parent cell divides in the absence of mitotic recombination. Here I show the cell before and after it synthesizes new DNA, which results in the replication of the chromosomes. Note that formally, DNA synthesis is not part of mitosis. Instead, it is considered part of the cell cycle.
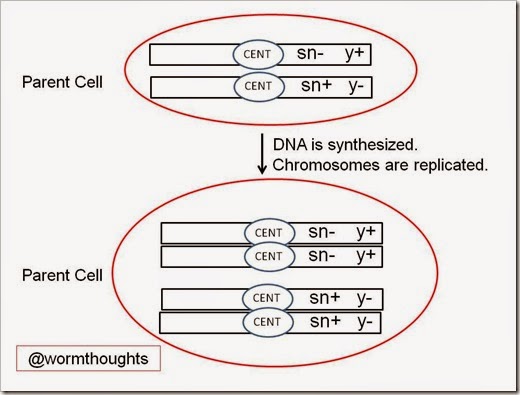
Now that we’ve replicated our homologous chromosomes, let’s follow them through mitosis and cytokinesis. Note that after the chromosomes have replicated, the sister chromatids are connected via the centromere (CENT in the figure). This organization will allow sister chromatids to be separated during mitosis and pulled apart into the nuclei of the two daughter cells. The dotted line below represents cytokinesis, or the dividing of the parent cell’s cytoplasm into two daughter cells.
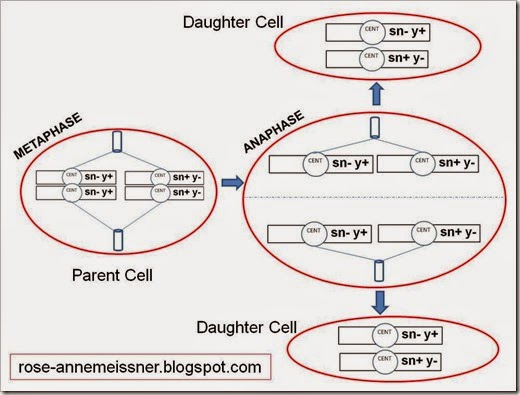
Note that after this cell divides, the daughter cells would have the same genotype (sn- y+/sn+ y-). Note that all of these cells would show a wild-type phenotype because each cell has a wild-type copy of sn and y.
Now, let’s consider a situation where mitotic recombination occurs. Scientists aren’t really sure when mitotic recombination happens. Some think it happens during interphase; that’s what I have shown below (specifically, Gap 2 after DNA synthesis is completed). The take-home point: mitotic recombination will switch the order of specific sn and y alleles compared to the original parent cell.
First I’ll show the recombination step and the reordered chromosomes. Note that recombination is taking place between chromosomes 2 and 3. Also note that the recombination breakpoint is between the centromere and the singed locus.

Now we have paired chromatids that have different genotypes. Chromatids 1 and 2 no longer match, but as you’ll see below, they will segregate during mitosis as though they are genetically identical. The same thing is true for chromatids 3 and 4.
Let’s follow the chromosomes through mitosis.
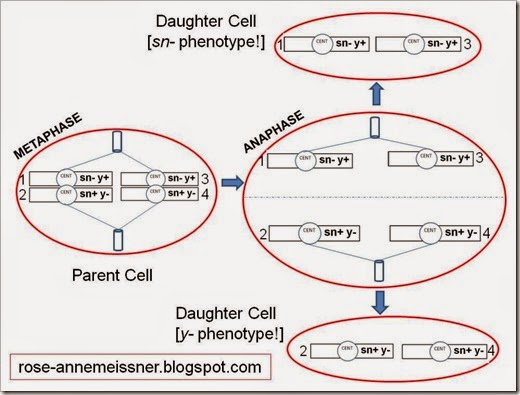
Because of the recombination event that happened during Gap 2, we end up with daughter cells that have different genotypes. Instead of the sn- y+/sn+ y- genotype that the parent cell has, our daughter cells are as follows:
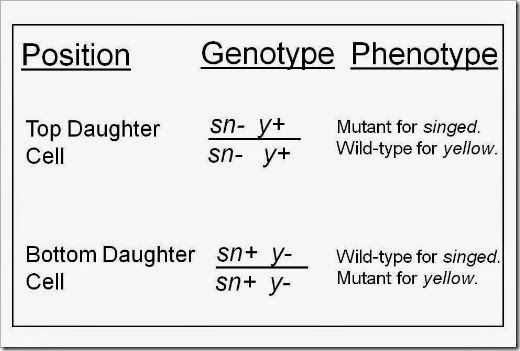
And finally, here is a cartoon to show how mitotic recombination in a precursor or parent cell could give rise to a twin spot. The idea here is after the parent cell undergoes mitotic recombination, the new daughter cells replicate themselves and make small populations that we can see visibly as the yellow or singed spots. (Pardon the reverse orientation on the spots; here I’ve shown the yellow spot on top and the singed spot on the bottom.)

References:
1Stern, C (1935) The effect of yellow-scute gene deficiency on somatic cells of Drosophila. Proc Natl Acad Sci USA 21: 374-379. {Find the full-length paper here.}
For more learning, I like the following links:
* Mosaic Analysis [in Drosophila]
* A good review of what happens to chromosomes during the cell cycle and mitosis
* * *
Like what you just read? To connect with me, you can find me on Twitter (I’m @wormthoughts) or by e-mail (r-meissner@u.northwestern.edu). Or leave a comment below!
Thanks for stopping by!